Syngeneic Mouse Models
Investigate cancer drugs in an immunologically intact environment to better assess immune responses and immunotherapy efficacy.
- Preclinical Oncology CRO Services | Clinical Trial Specialty Testing
- Syngeneic Models
Syngeneic Tumor Models: A Rapid System for Preclinical Immune Oncology Testing
Syngeneic mouse models are optimal for preclinical testing of innovative immune-oncology (IO) therapeutics within a fully functioning, intact immune system. Champions offers a collection of established syngeneic models with endogenous mouse therapeutic targets or genetically modified to express specific human genes.
-
A collection of models representing all relevant tumor indications
-
Fast turnaround times, compatible with a variety of endpoint analyses to fully characterize the therapeutic response and mechanism
-
Deep molecular characterization and response to Standard of Care data available
Well-characterized Response to Checkpoint Inhibitors
Champions has a wide range of well-characterized syngeneic mouse models with responses to immune checkpoint inhibitors, such as anti-PD-1 and anti-CTLA-4.
This platform can be used to assess efficacy or benchmark therapeutic agents against or in combination with standard of care immune therapies in an intact murine immune system.
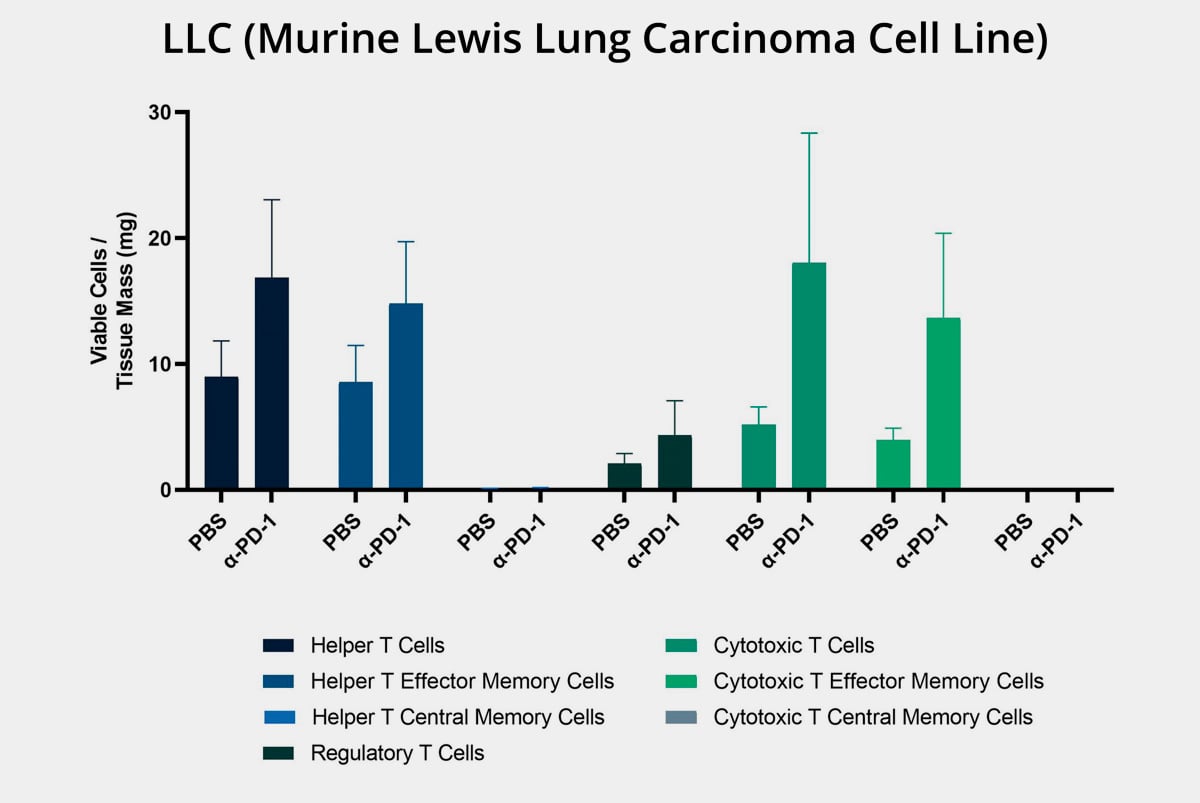
Additionally, these models can help you investigate your agent mechanism of action by measuring differences in immune cell population before and after treatment.
Syngeneic Models - Treated & Untreated Tumor Growth Curves
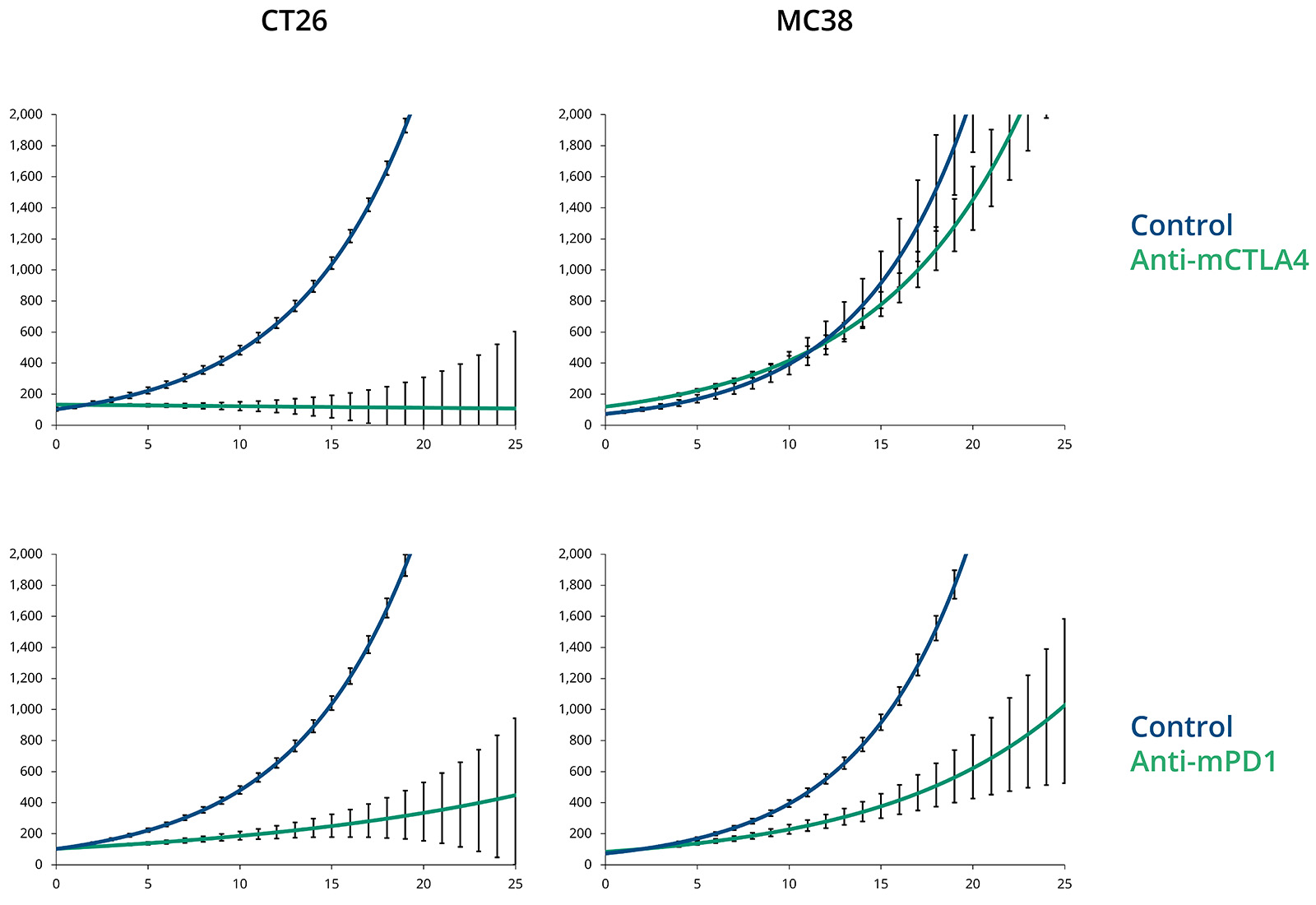
CT26 responds to both anti-mCTLA4 and anti-mPD1 in vivo, while MC38 responds to anti-mPD1 but not anti-mCTLA4. Plots show mean±SEM.
Champions Syngeneic Tumor Models
Champion's syngeneic mouse models offer key advantages for cancer research by using mice with a fully functional immune system to study immune responses in a natural context. These models replicate human immune system reactions, preserve the tumor microenvironment, and provide accurate insights into tumor-immune interactions. Cost-effective, reproducible, and versatile, they are ideal for testing immuno-oncology therapies, a wide range of tumor types, and assessing both innate and adaptive immune responses.
-
Cost-effective, large-scale syngeneic studies across multiple cell lines, including MC38, CT26, LLC, 4T1, EMT6, B16F10, RENCA, A20, KLN205, and MyC-CaP with key mutation and clinical characteristics.
-
An ideal platform for evaluating both innate and adaptive immune responses crucial for understanding immunotherapy effectiveness.
-
Flexible and versatile, enabling the study of various tumor types and testing of novel immunotherapies and combination therapies.
Syngeneic Model Endpoints


Syngeneic mouse models are preclinical in vivo models used to evaluate therapeutic candidates in mice with intact immune system. Humanized mouse models support the engraftment of many functional components of the human immune system. This Quick Guide gives a comparison of the two models and weighs the advantages and challenges of these models for oncology research.
What are syngeneic mouse models?
Syngeneic mouse models are in vivo models in which tumor cells from the same genetic background as the host mouse are implanted, allowing the study of cancer in an immunocompetent environment. These models are used to assess immune responses, evaluate cancer therapies, and study tumor-immune interactions in a way that closely mimics the human immune system's reaction to cancer treatments.
How do syngeneic models differ from xenograft models?
Syngeneic models and xenograft models differ primarily in the immune system of the host and the source of the tumor cells:
-
Immune System:
-
Syngeneic models use immunocompetent mice, meaning they have a fully functional immune system that can interact with the implanted tumor cells, allowing for the study of immune responses and immunotherapies.
-
Xenograft models use immunodeficient mice, which lack an adaptive immune system, preventing rejection of human tumor cells and making them suitable for studying human cancer cells in an immune-free environment.
-
-
Tumor Source:
-
Syngeneic models involve implanting tumor cells from the same genetic background as the host mouse, which maintains tumor-host immune interactions.
-
Xenograft models involve implanting human tumor cells into mice, which allows for the study of human cancer biology but lacks a functional immune response, limiting immune-related studies.
-
-
Applications:
-
Syngeneic models are ideal for testing immunotherapies and studying immune-tumor interactions.
-
Xenograft models are better suited for testing human-specific therapies and understanding human tumor behavior without the confounding effects of an immune system.
-
In summary, syngeneic models are used for studying cancer in an intact immune system, while xenograft models are used to study human tumors in an immunodeficient environment.
What are the advantages of using syngeneic models in immuno-oncology research?
Advantages of using syngeneic models in immuno-oncology research include:
Immunocompetent Environment: Syngeneic models use mice with a fully functional immune system, allowing for the study of immune responses to cancer and immunotherapies in a natural, intact immune context.
Relevance to Human Immunotherapy: These models closely mimic how the human immune system would respond to cancer therapies, making them highly relevant for testing immuno-oncology treatments.
Tumor-Immune Interaction: By preserving the native tumor microenvironment, syngeneic models provide valuable insights into tumor-immune interactions and the mechanisms of immune evasion.
Cost-Effectiveness: Syngeneic models are cost-effective and reproducible, offering a practical approach for preclinical testing and drug development.
Versatility: They can be used to study a wide variety of tumor types, evaluate novel immunotherapies, and test combination treatments in a consistent and standardized manner.
Evaluation of Immune Responses: These models are ideal for assessing both innate and adaptive immune responses, which are crucial for understanding the effectiveness of immunotherapies and developing new treatments.
Personalized Immuno-Oncology Research: Since they maintain the immune system’s integrity, syngeneic models are useful for testing immunotherapies in the context of tumor-specific immune responses, which can be aligned with patient-specific treatments.
These advantages make syngeneic models an essential tool in advancing immuno-oncology research and the development of targeted cancer therapies.
How are syngeneic models selected for specific cancer studies?
Syngeneic models are selected for specific cancer studies based on several factors:
-
Cancer Type: The model is chosen to match the cancer type under investigation. Various syngeneic tumor cell lines, such as CT26 for colon cancer, B16F10 for melanoma, or 4T1 for breast cancer, are used to closely replicate the human cancer type being studied.
-
Immune System Compatibility: As syngeneic models utilize mice with intact immune systems, the selected strain should exhibit immune responses relevant to the cancer type. This allows for the evaluation of immune responses and immunotherapies in a more natural setting.
-
Tumor Growth Dynamics: Models are selected based on their tumor growth rate, invasive behavior, and metastatic potential, aligning with the goals of the study, such as evaluating early-stage therapies or studying metastasis.
-
Therapeutic Relevance: The chosen syngeneic model should be compatible with the therapy being tested, whether it’s immunotherapy, chemotherapy, or combination treatments. Some models are better suited for specific therapies, like immune checkpoint inhibitors, while others are more appropriate for traditional cancer treatments.
-
Research Focus: The model selection also depends on the research focus, such as studying immune responses, the tumor microenvironment, or metastasis. Different models offer varying degrees of complexity in immune-tumor interactions.
Considering these factors ensures that syngeneic models are selected to effectively represent the cancer being studied and to align with the specific research and therapeutic goals.