Non-Small Cell Lung Cancer (NSCLC) In Vivo Screen
Evaluate your Non-Small Cell Lung Cancer (NSCLC) therapeutics on an extensive bank of carefully curated NSCLC patient-derived xenograft (PDX) models, including critical EGFR and KRAS mutant models.
Advance your Non-Small Cell Lung Cancer (NSCLC) therapeutics with our premier NSCLC In Vivo Screen.
Our screen offers an extensive biobank of solid tumor PDX models to test the effectiveness of your NSCLC therapeutics, with many models previously treated with the latest targeted therapies, checkpoint inhibitors, and EGFR-targeted treatments, closely reflecting real-life patient responses. Contact us today to advance your non-small cell lung cancer drug development project.
NSCLC In Vivo Screen offers:
- Comprehensive model characterization (stored in Lumin) with:
☑️Robust clinical data annotations
☑️NGS (RNAseq and WES) analysis
☑️Proteomics and phospho-proteomics - 50% off of a Standard of Care agent arm (selected by Champions)
- No minimum number of models to enroll and you have the ability to select the models you want to screen.
- Terminal tumor collections for target validation are available upon request (Snap Frozen/FFPE).
- Several endpoint data options available including Flow Cytometry and IHC to quantify target expression and critical phenotypical changes (available upon request).
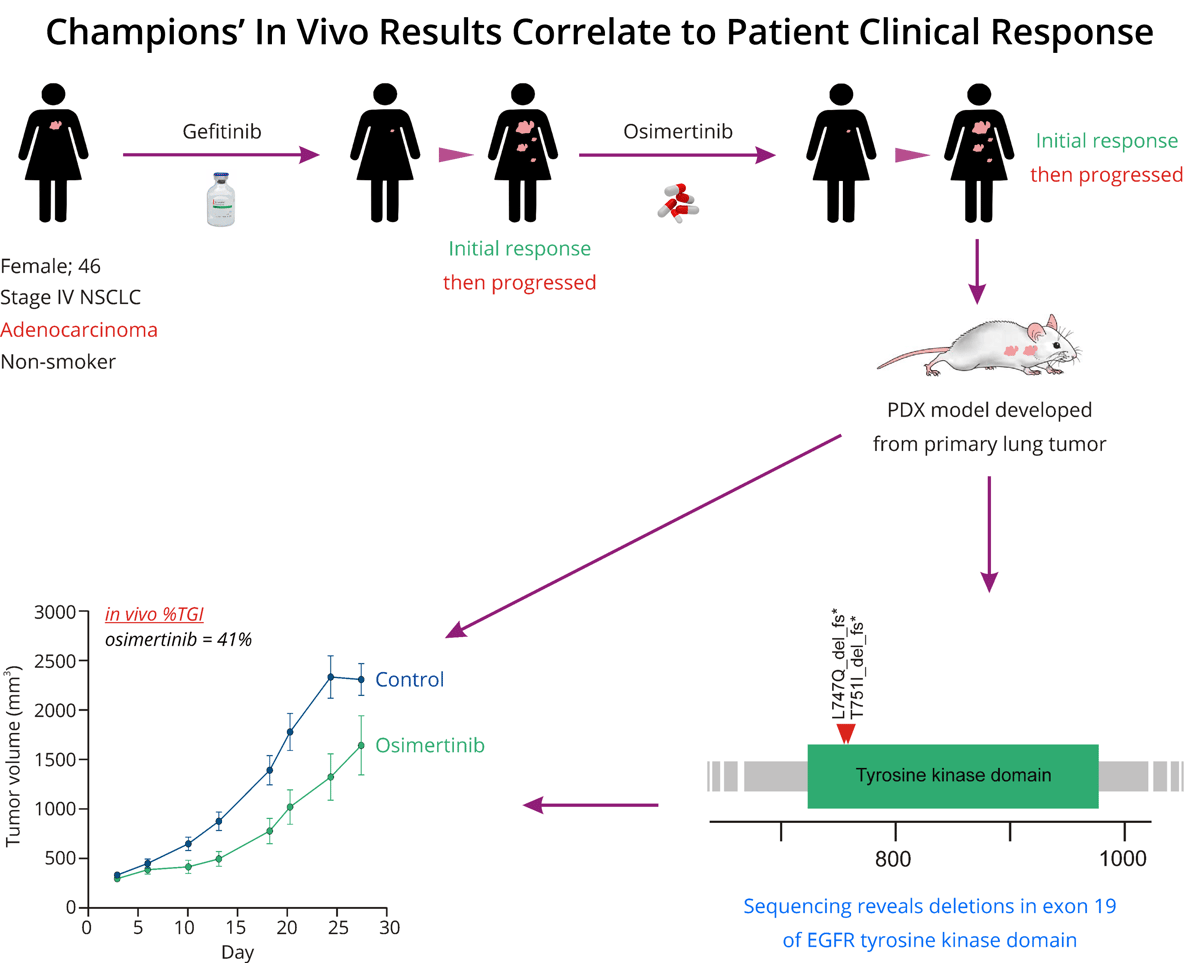
Figure 1: The workflow demonstrates a correlation between the results obtained from the NSCLC In Vivo Screen PDX model, developed from a primary lung tumor, and the patient's clinical response from the tumor.
