Humanized Mouse Models for Immuno-Oncology Research
Cutting-edge humanized mouse models tailored to harness the power of the human immune system and unveil the full potential of your preclinical immunotherapy research
- Preclinical Oncology CRO Services | Clinical Trial Specialty Testing
- Humanized Mouse Models for Immuno-Oncology Research
Champions’ humanized mouse tumor models provide a powerful platform for studying cancer in a controlled environment. With the largest fully characterized clinically relevant bank of PDX models in the industry and access to cutting-edge humanized mouse models, Champions is uniquely positioned to accelerate the development off immuno-oncology therapies. These models, developed through advanced techniques in humanization, help bridge the gap between xenograft vs syngeneic studies, offering more precise insights into the effectiveness of immuno-oncology treatments
Superior Humanized PDX Models and CDX Models
Champions Oncology supports your preclinical immuno-oncology (IO) research by providing, beyond the basic syngeneic models, cutting-edge humanized mouse model solutions specific for human immune targets. Multiple options are available to investigate and harness the human immune system in your studies:
-
Engraftment of cell therapies such as CAR T, CAR B, CAR NK, CAR macrophages, and DCs into the murine host
-
Adoptive transfer of Human PBMCs and NK cells in our PBMC ImmunoGraft® and NK ImmunoGraft® models: ideal for antibody-based therapeutics, and agents targeting T cells or NK cells
-
Engraftment of CD34+ hematopoietic stem cells from cord blood for full reconstitution of the human immune system in our CD34 Cell ImmunoGraft®
Preferred Platform for Preclinical Immuno-Oncology Drug Testing
Humanized mouse models are the preferred preclinical platform to test IO drug candidates in vivo.
By leveraging different transgenic mouse strains, we provide access to mouse models reconstituted with a fully functional human immune system including T and B lymphocytes, myeloid cells, macrophages, DCs, and NK cells. In partnership with TransCure BioServices, we offer customizable gene delivery through hydrodynamic boosting, which promotes differentiation into different human immune populations.
This innovative platform allows clients to evaluate the efficacy, pharmacodynamics, and mechanisms of action of various cancer therapeutics that modulate human immunity, such as checkpoint inhibitors, monoclonal and bispecific antibodies, cancer vaccines, cytokine-based therapies, and combinatorial treatments.
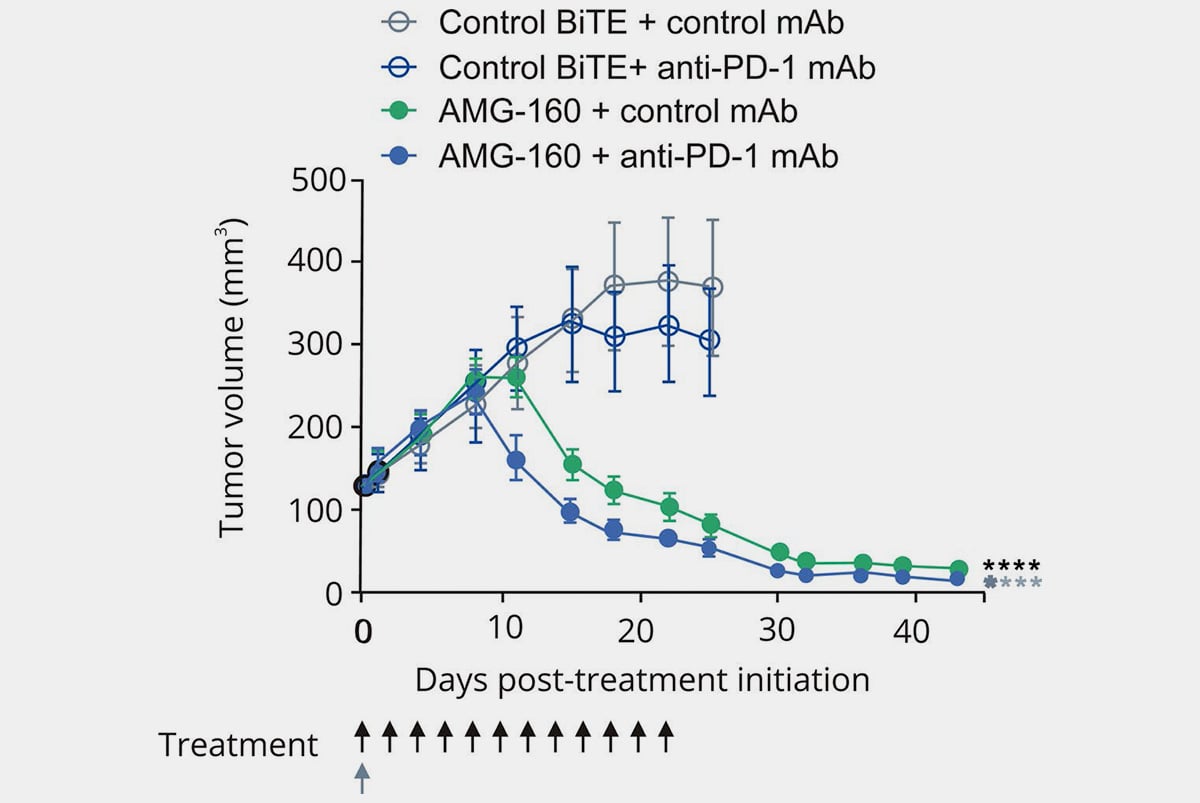
Humanized Mouse Model Endpoints














Syngeneic mouse models are preclinical in vivo models used to evaluate therapeutic candidates in mice with intact immune system. Humanized mouse models support the engraftment of many functional components of the human immune system. This Quick Guide gives a comparison of the two models and weighs the benefits and challenges of these models for oncology research.
Humanized mouse models are models that support the engraftment of many functional components of the human immune system. These mouse models have various human immune cells engrafted and can be used to evaluate therapeutic candidates in a variety of human diseases. Read our Humanized Mouse Model Platform fact sheet to learn more about this platform.
Mouse models have been the workhorses of preclinical immuno-oncology (IO) research, and advances in mouse model development have expanded to applications for nearly all types of solid tumors and hematological malignancies. Here we highlight features of syngeneic and humanized mouse models and define which models are most relevant to different phases of preclinical IO research.
Ready to start working with Champions Oncology?
Unlock the power of humanized immune system mice to drive your oncology research forward. Champions Oncology offers advanced humanized PDX models that closely replicate human tumor-immune interactions, providing unparalleled insights into immunotherapy efficacy. Let us partner with you to accelerate your drug development and cancer research.
How Do Humanized PDX Models Differ from Traditional PDX Models?
Humanized Patient-Derived Xenograft (PDX) models differ from traditional PDX models because they incorporate a functional human immune system within immunocompromised mice that host the patient-derived tumors. Traditional PDX models involve implanting human tumor tissues into mice, but these models lack a human immune response, limiting their use in studying immune-based therapies. In contrast, humanized PDX models engraft human immune cells into the mice, allowing the interactions between human tumors and the immune system. This enhancement makes humanized PDX models particularly valuable for research in immuno-oncology, for the evaluation of immune-mediated side effects, and for the development of immunotherapies.
What Services Are Included with Humanized PDX Model Development?
Humanized PDX model development services typically include:
- Human Immune System Reconstitution: The introduction of human immune cells, such as hematopoietic stem cells or peripheral blood mononuclear cells (PBMCs), into the mice.
- Testing different immune cell donors: Leveraging different donors allows accounting for donor variability.
- Tumor Implantation: Implanting patient-derived tumor tissues into the humanized mice.
- In Vivo Monitoring: Regular assessment of tumor growth and immune system activity within the model.
- Data Collection and Analysis: Comprehensive evaluation of tumor-immune interactions, including immune cell profiling, cytokine, Immuno-histochemistry, body weight, and treatment response (Tumor growth Inhibition).
How Long Does It Take to Develop a Humanized PDX Model?
The development of a humanized PDX model can take several months. The process begins with humanizing the mice by engrafting them with human immune cells, which typically takes several weeks. After successful engraftment, patient-derived tumor tissues are implanted into the mice. The tumor needs additional time to grow and interact with the humanized immune system. Overall, the process from humanization to a fully developed and validated model can take between 3 to 6 months, depending on the specific requirements of the study and the tumor growth kinetics. Champions, through fully optimized workflows and continuous access to humanized mice, can offer much shorter timelines to achieve your goals faster.
How Are Humanized PDX Models Validated?
Humanized PDX models are validated through several key steps:
- Immune Cell Engraftment Assessment: Confirming the presence and functionality of human immune cells within the model.
- Tumor Growth Monitoring: Observing the behavior and progression of the tumor in the presence of the human immune system to ensure it mirrors human disease.
- Treatment Response Testing: Evaluating how the model responds to therapies to ensure it accurately predicts clinical outcomes.
- Histopathological and Molecular Analysis: Conducting detailed tissue analyses to validate immune cell infiltration, tumor structure, and molecular signatures within the model.
What Types of Data Can Be Generated from Humanized PDX Models?
Humanized PDX models can generate a wide array of data, including:
- Survival curves: survival experiments generating Kaplan Meyer curves comparing different treatments
- Tumor Growth Data: Metrics on tumor size and response to treatments.
- Immune Profiling Data: Detailed information on the types and activity of human immune cells within the tumor microenvironment.
- Pharmacokinetic and Pharmacodynamic Data: Insights into how drugs are metabolized and retained in the model and their effects on both the tumor and immune system.
- Histopathological Data: Microscopic analysis of tumor tissues, including immune cell infiltration and tumor architecture.
- Molecular Data: Information on gene expression, protein levels, and genetic mutations within the tumor and surrounding immune cells, aiding in the understanding of treatment mechanisms and resistance.
"Champions' humanized PDX models have given us a highly relevant platform for studying human-specific tumor interactions, which has been critical for evaluating our therapeutic approaches and optimizing treatment strategies."
___________________________________________________
"The biologically relevant tumor microenvironment in Champions' humanized models has greatly enhanced our ability to assess drug responses and uncover novel therapeutic targets."
___________________________________________________
"Champions' humanized PDX models have given us a highly relevant platform for studying human-specific tumor interactions, which has been critical for evaluating our therapeutic approaches and optimizing treatment strategies."
___________________________________________________
"The biologically relevant tumor microenvironment in Champions' humanized models has greatly enhanced our ability to assess drug responses and uncover novel therapeutic targets."
___________________________________________________
"Champions' humanized PDX models have given us a highly relevant platform for studying human-specific tumor interactions, which has been critical for evaluating our therapeutic approaches and optimizing treatment strategies."
___________________________________________________
"The biologically relevant tumor microenvironment in Champions' humanized models has greatly enhanced our ability to assess drug responses and uncover novel therapeutic targets."
___________________________________________________