Early Drug Discovery Services
Empowering early-stage discovery with proprietary platforms designed for fast and precise identification of cancer therapeutic targets.
- Preclinical Oncology CRO Services | Clinical Trial Specialty Testing
- Early Drug Discovery Services

Rapid Identification of Therapeutic Targets
Champions' Early Drug Discovery services provide a rapid and cost-effective solution to propel your discovery projects forward with reliable data and confidence. To advance your integrated drug discovery research, Champions provides two proprietary integrated workflows:
-
ChemiSelect: Our proprietary small molecule assay platform, developed to identify and prioritize chemotypes effectively.
-
ADC-Flow: Our proprietary platform to screen and engineer the most effective Antibody Drug Conjugates (ADC) tailored precisely to your target requirements.
ChemiSelect: Prioritize Chemotypes for Difficult-to-Assay Targets
Champion’s ChemiSelect offers an integrated drug discovery workflow engineered for the functional characterization of chemotypes within physiologically relevant intracellular environments. This facilitates the selection of the most potent cytotoxic compounds for your therapeutic targets, utilizing clinically relevant cell lines.
-
Cell-Based Platform: Prioritize chemotype selection and conduct SAR for challenging targets within an intracellular setting, ensuring authentic target binding despite complexities such as cellular proteins and unknown cofactors, thus ensuring confidence in selectivity, affinity, and binding.
-
Economical: Offers cost-effective solutions with fast turnaround times, saving valuable resources.
-
Mitigate Liabilities: The screen addresses ADMET concerns, including issues with permeability, stability, and solubility, commonly linked to targets in the later stages of drug development.

ChemiSelect Integrated Workflow
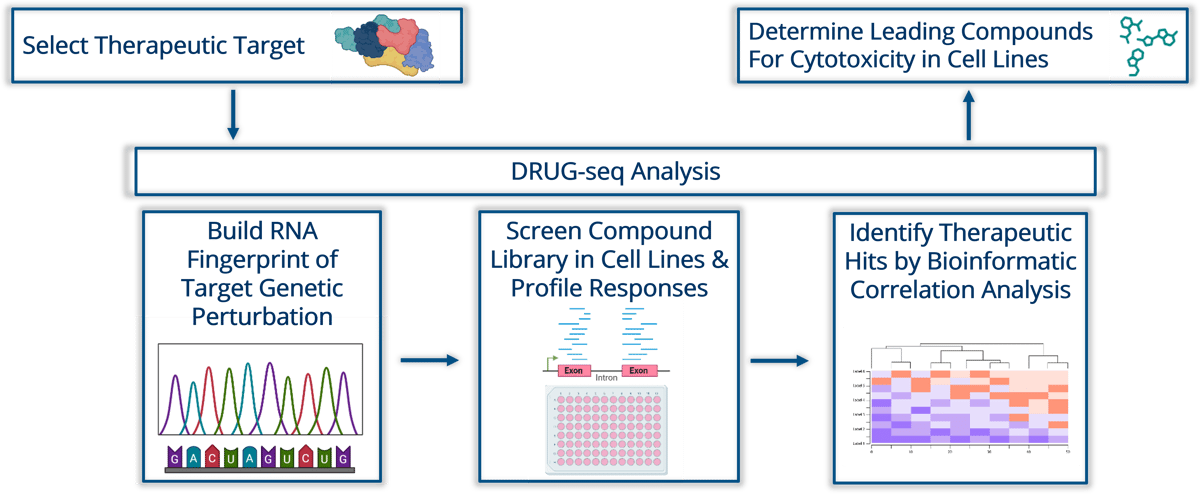
Figure A: The ChemiSelect Integrated drug discovery Workflow outlines the successive stages of the screening process. Employing our advanced DRUG-seq analysis platform, we systematically assess the therapeutic target. This entails building genetic perturbations of the target, followed by compound screening in cell lines, and conducting bioinformatics analysis to pinpoint the most potent and effective cytotoxic compounds.
ADC-Flow: Develop & Validate Early Translational Strategies for ADC Therapeutics
Champions' ADC-Flow is designed to develop the most potent ADCs and evaluate the efficacy of antibodies and payload cytotoxicity, including potential off-target binding, during the early discovery phase, and minimizing clinical setbacks in later stages of drug development.
-
Formulate Translational Hypotheses through IHC: Uncover patient cohort hypotheses and establish early translational strategies for your oncology ADC program.
-
Screen & Select: Efficiently screen large antibody libraries through a rapid and cost-effective assay, selecting mAb hits with internalization and cancer cell-killing capabilities.
-
Validate Hypotheses via Ex Vivo Clinical Trial Simulations: Evaluate your ADCs within a clinically relevant patient cohort to gauge potential objective responses, considering expression threshold and payload sensitivity.
ADC-Flow Integrated Workflow
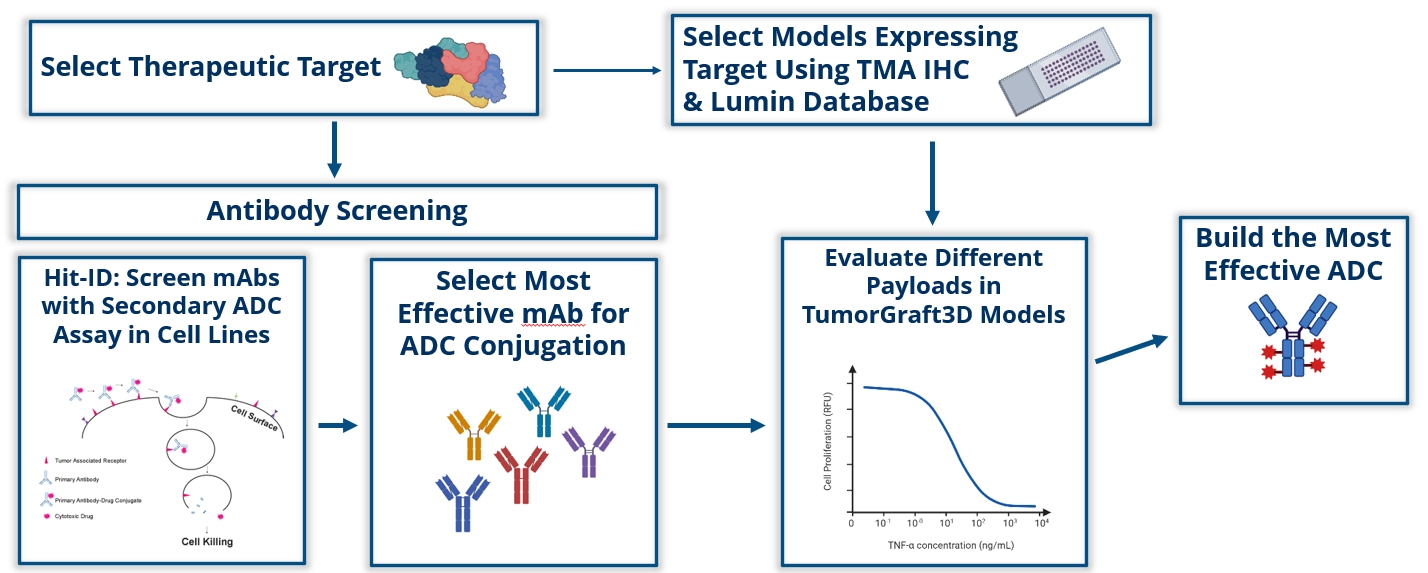
Figure B: The ADC-Flow integrated workflow delineates the sequential stages of the screening process. Leveraging our cutting-edge antibody analysis, we methodically assess mAbs using a secondary ADC assay in cell lines. This process facilitates the identification of the most efficacious mAb candidates for ADC conjugation. Subsequently, payload evaluation in TumorGraft3D models is conducted, resulting in results to design the most potent and effective ADC candidates.
What are the main stages of drug discovery?
Key Stages in Drug Discovery
The drug discovery process follows a systematic approach to identify, develop, and introduce new therapeutics to the market. The main stages include:
1. Target Identification & Validation - Recognizing a biological molecule (such as a protein or gene) that plays a role in a disease and confirming that modifying this target could have therapeutic benefits.
2. Hit Identification - Screening extensive chemical libraries to find compounds that interact with the identified target. Techniques used include high-throughput screening (HTS), computational (virtual) screening, and fragment-based drug discovery.
3. Lead Optimization - Refining selected hit compounds to enhance potency, selectivity, and pharmacokinetic properties that minimize toxicity while improving drug-like characteristics.
4. Preclinical Testing - Conducting laboratory and animal studies to evaluate safety, efficacy, and optimal dosing while assessing toxicity, metabolic behavior, and pharmacodynamics (the drug’s effects on the body).
5. Clinical Trials (Human Studies) - Clinical trials progress through multiple phases to evaluate safety and therapeutic effectiveness:
- Phase 1: Small-scale trials (20–100 healthy individuals) to determine safety and appropriate dosage.
- Phase 2: Mid-sized studies (100–500 patients) to assess efficacy and monitor side effects.
- Phase 3: Large-scale trials (1,000–5,000+ participants) to confirm effectiveness and track adverse reactions.
6. Regulatory Approval - Submitting comprehensive data to regulatory bodies (such as the FDA or EMA) for review, and upon approval, the drug becomes available for medical use.
7. Post-Market Monitoring (Phase 4) - Observing long-term safety, rare side effects, and real-world effectiveness, and conducting additional research for potential new applications or formulations.
How are potential drug targets identified?
From a drug discovery perspective, potential drug targets are identified by analyzing disease mechanisms and pinpointing biomolecules (proteins, genes, or pathways) that can be modulated for therapeutic benefit. This process involves genomic and proteomic studies, bioinformatics-driven target prediction, functional assays, RNAi and CRISPR screening, and high-throughput screening (HTS) using in vitro, ex vivo, and in vivo platforms. Once identified, targets undergo validation to confirm their role in disease progression and druggability.
What role does computational modeling play in drug discovery?
Computational modeling plays a critical role in drug discovery by accelerating target identification, optimizing drug design, and predicting drug-target interactions. It enables structure-based drug design (SBDD), ligand-based drug design (LBDD), virtual screening, and molecular docking to identify promising compounds. Additionally, AI-driven simulations and machine learning models help predict pharmacokinetics, toxicity, and drug efficacy, reducing the need for extensive laboratory testing and expediting the discovery process.
How is the safety and efficacy of a drug candidate evaluated in early stages?
In the early stages of drug discovery, the safety and efficacy of a drug candidate are evaluated through preclinical studies, which include:
In Vitro Testing – Conducted on cell cultures to assess the drug’s biological activity, toxicity, and mechanism of action.
In Vivo Studies – Performed on animal models to evaluate pharmacokinetics (absorption, distribution, metabolism, and excretion) and pharmacodynamics (drug effects on the body).
Toxicity Studies – Identify potential adverse effects, including acute, chronic, and genotoxicity assessments.
Dose-Response Studies – Determine the optimal dose range for therapeutic effects while minimizing toxicity.
If a drug shows promising safety and efficacy in preclinical studies, it progresses to clinical trials for further evaluation in humans.
