Clinical Kitting Services & Logistics
Tailored clinical kitting services to streamline your clinical trial.
- Preclinical Oncology CRO Services | Clinical Trial Specialty Testing
- Clinical Sample Collection Kits & Logistics
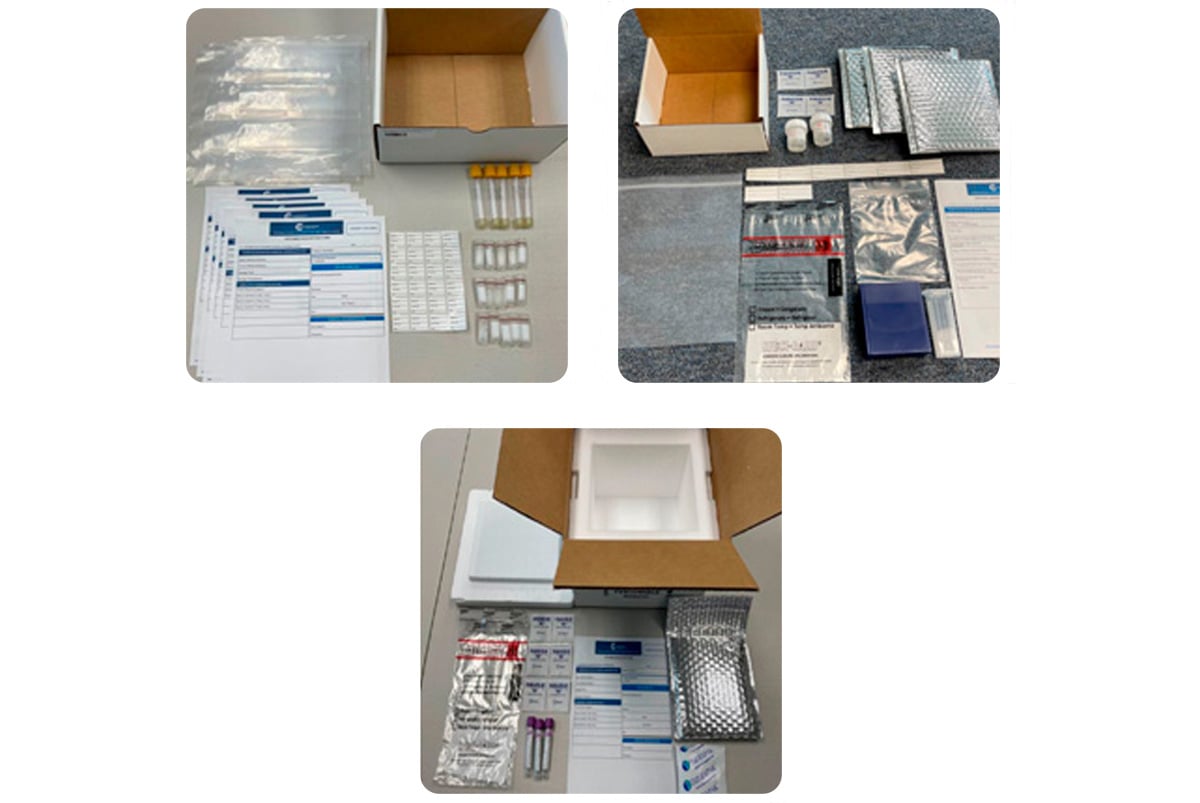
Clinical Kitting Services: Convenient Preparation of Sample Collection Kits
In addition to providing expert specialty testing and data analysis, Champions also creates custom test kits for your clinical trial.
-
We leverage our experience to work with you throughout the development and validation stages to include the best tube types and reagents in each kit
-
Streamline efforts to ensure your clinical sites have up-to-date inventory for each patient sample collected
-
Maintain constant communication between each clinical site and our clinical specialty testing laboratories
Champions Oncology’s clinical kitting services encompass the assembly, packaging, and distribution of customized kits for clinical trials. Each kit includes essential sample collection tools, such as specialized tubes, reagents, and necessary documentation, ensuring consistency and compliance with regulatory standards. Our experts collaborate throughout the development and validation stages to implement the best protocols for each kit. By optimizing logistics, maintaining strict quality control, and offering training when needed, our clinical kitting services enhance efficiency, minimize errors, and streamline the workflow of your clinical research.
-
Ease of Use – Pre-packaged tailored kits specifically assembled for your unique project needs with simple workflows, reducing setup time and minimizing errors.
-
Improved Accessibility – Our kits are readily available and customized for your study needs, ensuring all necessary components are already included.
-
Regulatory Compliance – Strict adherence to regulatory industry standards to ensure patient safety, data integrity and generate high-quality data.
-
Time and Cost Savings – Our processes eliminates the need for on-site kit assembly, allowing you to focus on trial execution.
-
Enhanced Efficiency: Standardized kits enhance efficiency by streamlining logistics, sample handling, and documentation while minimizing errors through clear labeling and strict protocol adherence.
-
Global Reach – Reliable distribution and supply chain protocols ensure kits are available wherever clinical studies are conducted.
Uniform Sample Management
Consistent sample handling across all trial sites, ensuring study integrity from collection to analysis.
Structured Site
Training
Detailed training programs for site staff to ensure standardized sample collection and minimized variability
Seamless Global
Logistics
Efficient logistics for your samples, ensuring smooth operations and the integrity of your clinical research
Clinical Site Training to Ensure Consistency
Champions understands that transferring protocols for sample collection is critical to obtaining high-quality data in your clinical trial. Champions also offers onsite face-to-face clinical site training as a service upon request.
We want to guarantee your samples are collected properly and consistently from the first patient to the last, as the data you receive is only as good as the sample collected initially.
Our expert team will train your clinical sites globally to ensure that each sample collected follows the proper applicable collection protocol and that the kit is prepared and shipped according to standard operating procedures.

What is clinical kitting in the context of clinical trials?
In the context of clinical trials, clinical kitting refers to the process of assembling, packaging, and distributing pre-configured kits containing all necessary materials for sample collection, processing, and transportation. These kits are customized to meet study-specific requirements and typically include items such as collection tubes, swabs, reagents, labeling, documentation, and shipping materials.
Clinical kitting ensures standardization across trial sites, improves efficiency, reduces errors, and maintains regulatory compliance (e.g., FDA, GMP, ISO). By streamlining logistics and providing clear protocols, clinical kitting enhances the accuracy and reliability of data collection, ultimately supporting the success of clinical research.
Importance of Clinical Kitting in Trials
-
Standardization Across Sites – Ensures all study sites follow the same collection and handling procedures, reducing inconsistencies in data.
-
Regulatory Compliance – Adheres to global standards such as FDA, GMP, and ISO to ensure the safety and integrity of clinical samples.
-
Improved Efficiency – Pre-assembled kits streamline trial operations, allowing researchers to focus on study execution rather than logistics.
-
Error Reduction – Clear labeling, detailed protocols, and standardized materials minimize the risk of sample mix-ups or incorrect collection.
-
Time and Cost Savings – Reduces the need for site-specific kit assembly and decreases logistical delays, improving overall study timelines.
-
Global Distribution & Accessibility – Clinical kits can be shipped worldwide, ensuring that research teams have timely access to essential materials.
How Clinical Kitting Supports Trial Success?
By centralizing the preparation and distribution of clinical kits, sponsors and CROs (Contract Research Organizations) can ensure that all study sites receive consistent, high-quality materials. This improves the reliability of trial results and supports regulatory approval by maintaining sample integrity and data accuracy throughout the study.
